


Copyright@2011 University of South Carolina

Organic chemistry
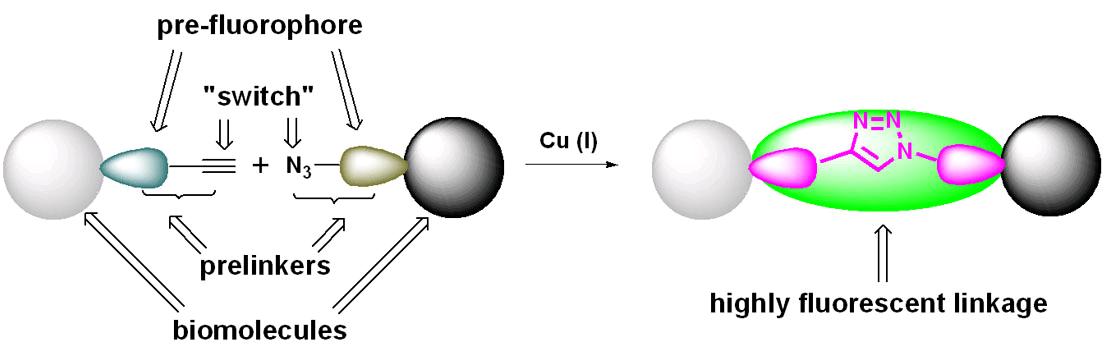
Fluorescence generated by "click"
We have developed a series of fluorogenic CuAAC reactions based on coumarin, anthracene and BODIPY fluorocores. In addition to being used in the combinatorial synthesis of fluorescent dyes, the most important application of these reactions is the bioconjugation and bioimaging within the intracellular environment. For example, incorporation of exogenous natural or unnatural tags into proteins or glycans by cellular biosynthetic pathways is an emerging strategy for investigating their cellular activities. Since those processes involve multistep enzymatic transformations that prohibit the incorporation of large signaling moieties, chemoselective reactions are often employed for post-labeling. In this case, a bioorthogonal fluorogenic reaction is invaluable, in which the unreacted reagents show no fluorescent background and the purification process can be circumvented. One of the 3-azidocoumarins developed in our group had been successfully utilized for in vivo protein labeling study in collaboration with the D. Tirrell group (CalTech).
We have developed a series of fluorogenic CuAAC reactions based on coumarin, anthracene and BODIPY fluorocores. In addition to being used in the combinatorial synthesis of fluorescent dyes, the most important application of these reactions is the bioconjugation and bioimaging within the intracellular environment. For example, incorporation of exogenous natural or unnatural tags into proteins or glycans by cellular biosynthetic pathways is an emerging strategy for investigating their cellular activities. Since those processes involve multistep enzymatic transformations that prohibit the incorporation of large signaling moieties, chemoselective reactions are often employed for post-labeling. In this case, a bioorthogonal fluorogenic reaction is invaluable, in which the unreacted reagents show no fluorescent background and the purification process can be circumvented. One of the 3-azidocoumarins developed in our group had been successfully utilized for in vivo protein labeling study in collaboration with the D. Tirrell group (CalTech).
•
Org. Lett., 2004, 6, 4603
J. Am. Chem. Soc., 2005, 14582
Angew. Chem. Int. Ed., 2006, 7364
Lett. Org. Chem. 2006, 3, 35
Tetrahedron 2008, 64, 2906
Tetrahedron Lett. 2009, 50, 7032
BioTechniques, 2010, 49, 525
Science China. 2010, 53, 1287
J. Am. Chem. Soc., 2005, 14582
Angew. Chem. Int. Ed., 2006, 7364
Lett. Org. Chem. 2006, 3, 35
Tetrahedron 2008, 64, 2906
Tetrahedron Lett. 2009, 50, 7032
BioTechniques, 2010, 49, 525
Science China. 2010, 53, 1287
a fluorogenic CuAAC reaction was introduced for labeling and detection of DNA in proliferating cells.


