


Copyright@2011 University of South Carolina

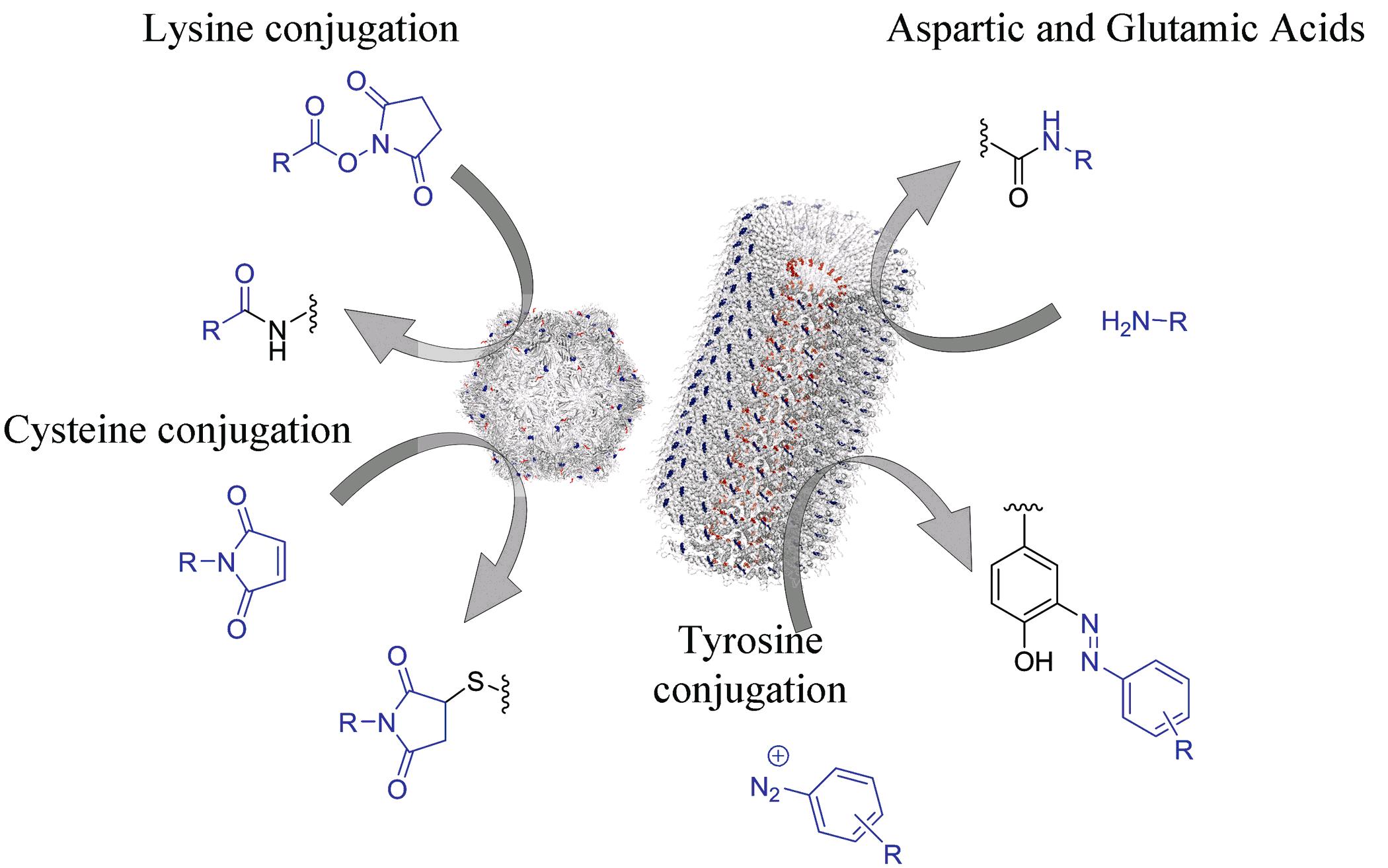
Bioconjugation
Bioconjugate small molecules onto virus surface
Viruses and virus-like particles have emerged over recent years as promising building blocks for chemical reactions and materials syntheses. These bio-inspired systems form monodispersed units that are highly amenable through genetic and chemical modifications. As nano-scale assemblies, viruses have sophisticated yet highly ordered structural features, which, in many cases, have been carefully characterized by modern structural biological methods. Therefore, viruses offer a unique scaffold where functional motifs can be programmed on their coat proteins precisely at sub-nanometer scale via bioconjugation recognition, which is a big advantage over synthetic nanoparticles.
Viruses and virus-like particles have emerged over recent years as promising building blocks for chemical reactions and materials syntheses. These bio-inspired systems form monodispersed units that are highly amenable through genetic and chemical modifications. As nano-scale assemblies, viruses have sophisticated yet highly ordered structural features, which, in many cases, have been carefully characterized by modern structural biological methods. Therefore, viruses offer a unique scaffold where functional motifs can be programmed on their coat proteins precisely at sub-nanometer scale via bioconjugation recognition, which is a big advantage over synthetic nanoparticles.
•
(4) Chemical modification of M13 bacteriophage and its application in cancer cell imaging. The M13 bacteriophage has been demonstrated to be a robust scaffold for bionanomaterial development. We reported on the chemical modifications of three kinds of reactive groups, i.e., the amino groups of lysine residues or N-terminal, the carboxylic acid groups of aspartic acid or glutamic acid residues, and the phenol group of tyrosine residues, on M13 surface. In addition, cancer cell targeting motifs such as folic acid could also be conjugated onto the M13 surface. Therefore, dual-modified M13 particles with folic acid and fluorescent molecules were synthesized via the selective modification of two kinds of reactive groups. Such dual-modified M13 particles showed very good binding affinity to human KB cancer cells, which demonstrated the potential applications of M13 bacteriophage in bioimaging and drug delivery.

Here are some representative works:
(1) Dual modification of turnip yellow mosaic virus (TYMV) as a prototype BNP for time-resolved fluoroimmuno assay. TYMV is an icosahedral plant virus with an average diameter of 28 nm and can be isolated in grams quantities from turnip or Chinese cabbage inexpensively. Two types of reactive amino acid residues were employed to anchor luminescent terbium complexes and biotin groups based on orthogonal chemical reactions. While terbium complexes were used as luminescent signaling groups, biotin motifs were acted as a model ligand for protein binding. Furthermore, the Cu(l) catalyzed azide-alkyne cycloaddition (CuAAC) reaction, a prototype of "click" chemistry, have been used to post-functionalize TYMV to graft peptide and other ligands to promote or inhibit the cell binding properties.
(1) Dual modification of turnip yellow mosaic virus (TYMV) as a prototype BNP for time-resolved fluoroimmuno assay. TYMV is an icosahedral plant virus with an average diameter of 28 nm and can be isolated in grams quantities from turnip or Chinese cabbage inexpensively. Two types of reactive amino acid residues were employed to anchor luminescent terbium complexes and biotin groups based on orthogonal chemical reactions. While terbium complexes were used as luminescent signaling groups, biotin motifs were acted as a model ligand for protein binding. Furthermore, the Cu(l) catalyzed azide-alkyne cycloaddition (CuAAC) reaction, a prototype of "click" chemistry, have been used to post-functionalize TYMV to graft peptide and other ligands to promote or inhibit the cell binding properties.
Bioconj. Chem. 2007, 18, 852
J. Am. Chem. Soc. 2007, 129, 7799
Bioconjugate Chem. 2011, 22, 58-66
J. Am. Chem. Soc. 2007, 129, 7799
Bioconjugate Chem. 2011, 22, 58-66
Figure 1
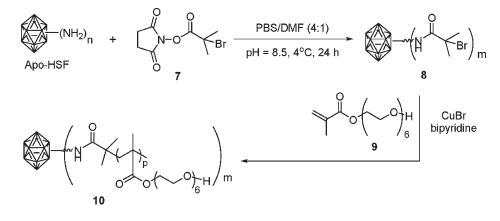
(2) Chemoselective modification of apoferritin. We reported the chemoselective modification of apoferritin using conventional bioconjugate chemistry, followed with CuAAC reaction and an in situ atom transfer radical polymerization reaction on the outer surface of apoferritin. These transformations afford versatile methods to alter the properties of apoferritin particles, and can be extended to other BNPs.
Figure 2
(3) Extremely efficient conjugation of tobacco mosaic virus (TMV) using CuAAC reaction. TMV is a rod-shaped, 300 nm long and 18 nm in diameter, with a central hold of diameter of 4 nm to encapsidate RNA genome. TMV can be decorated based on the synergistic combination of a CuAAC reaction and an aryldiazonium coupling reaction. The combination of these reactions provides an extremely efficient way (>99.99% transformation for every single step) to conjugate a wide spectrum of compounds to the phenolic groups of tyrosine residues presented on the TMV capsid. To highlight the modularity and efficiency of this "click" conjugation, a cell adhesion assay was performed after TMV was modified with functionalities that can promote or prevent cell adhesion and proliferation.
ChembioChem 2008, 9, 519 (Cover Story)
Tobacco mosaic virus based thin film sensor for detection of volatile organic compounds. A thin film sensor for the detection of volatile organic compounds (VOC) was fabricated by deposition of oligo-aniline grafted tobacco mosaic virus (TMV) onto a glass substrate. The oligo-aniline motifs were conjugated onto the TMV surface by a traditional diazonium coupling reaction to tyrosine residues followed by Cu(I) catalyzed alkyne-azide cycloaddition (CuAAC) reaction. The modified TMV was easily fabricated into a thin film by directly drop coating onto a glass substrate. Upon integration of the glass substrate into a prototypical device, the virus-based thin film exhibited good sensitivity and selectivity toward ethanol and methanol vapour.
J. Mater. Chem., 2010, 20, 5715 - 5719.
Bioconjugate Chem. 2010, 21, 1369-1377
Chem. Commun. 2007, 14, 1453


